From speculation to predictability
Enhanced patient safety isn’t just about strong testing - it comes from robust interfaces and risk management that goes beyond qualitative judgement. RD8 brings quantitative methods and traceability into risk assessment, turning documentation into real design control.
.png)
In regulated processes, risk management is often qualitative and documentation-heavy. That creates dependency on individual risk experts and weakens the link to specifications. The result: limited impact, missed interactions, and unclear traceability.
RD8’s quantitative approach to risk assessment and interface risk
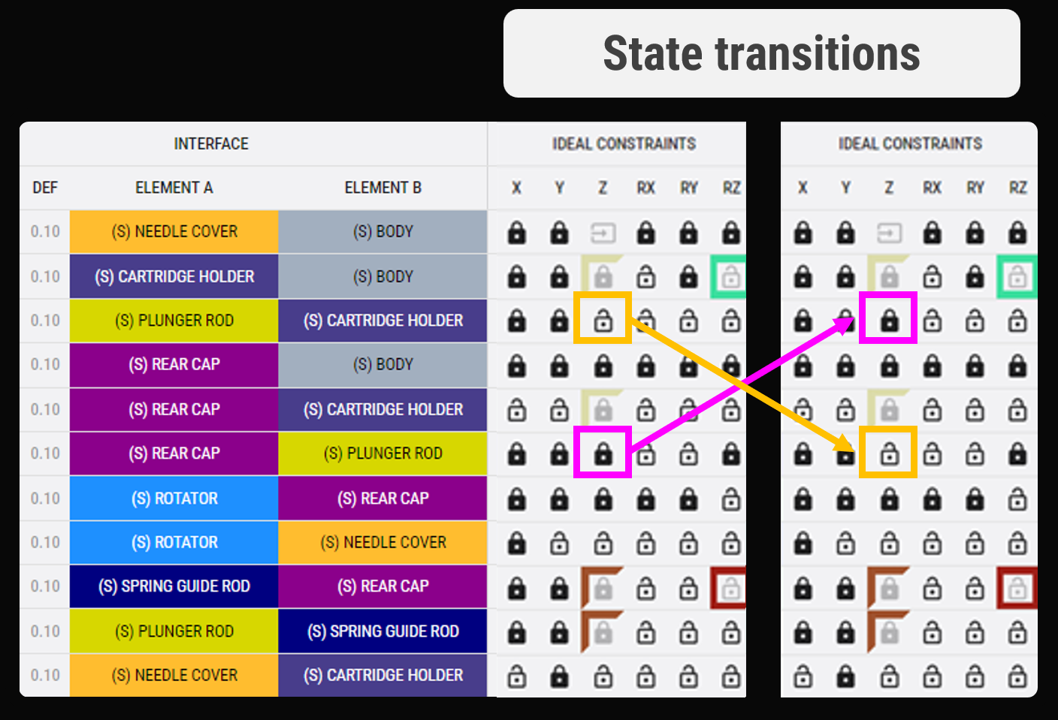
Predictability at the interface level
We identify and quantify unwanted mechanical interactions with a bottom-up process.
Rule-based checklists ensure no negative effects are overlooked, and identified risks are aggregated at system level and linked directly to requirements and specifications.
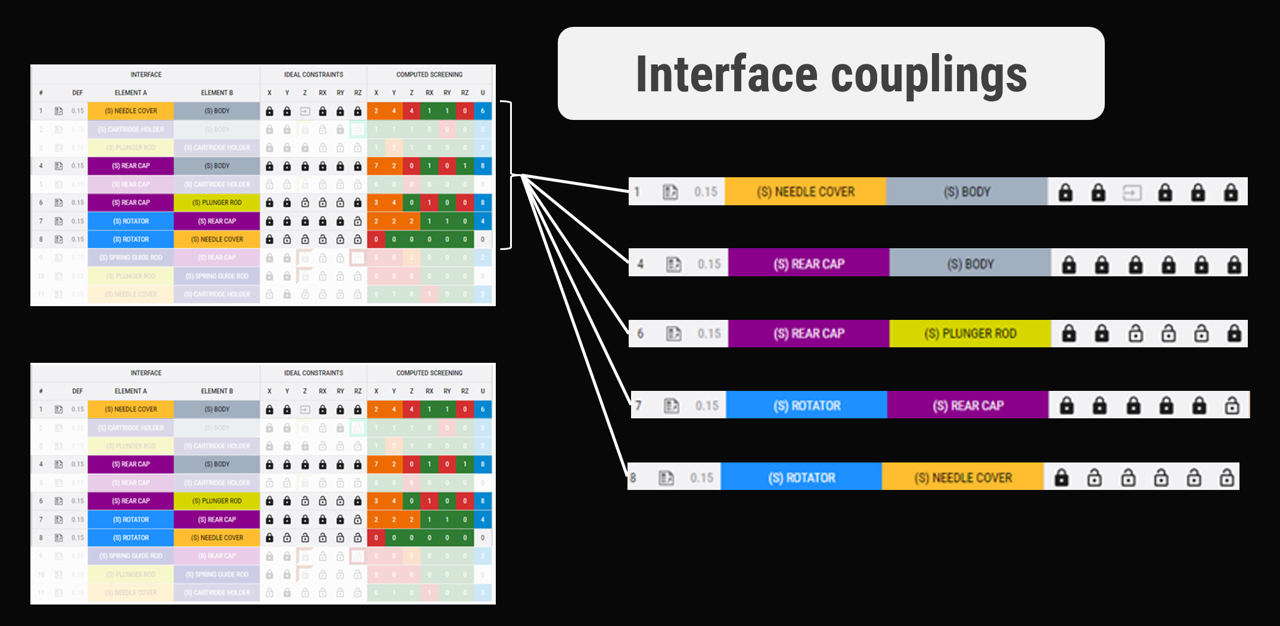
Couplings, dependencies, and traceability
Through a top-down architecture process, we establish clear interface specifications and maintain traceability across requirements, drawings, and mitigations.
This creates a direct link between risk management and real product design.
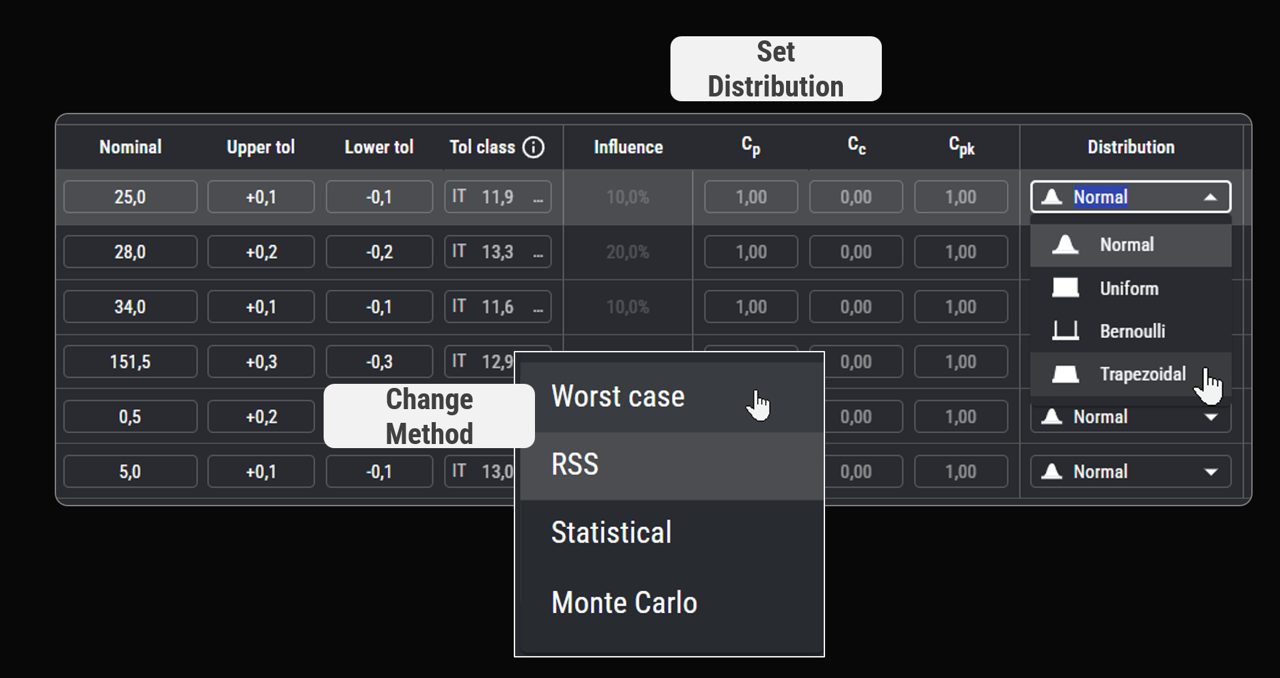
Dimensioning for capability
By combining tolerance allocation methods or real capability data, we link part specifications to rationales and control strategies.
This enables data-driven failure rates forecasts and makes risk control both verifiable and efficient.
Ready to get more out of your risk management process?
RD8 helps device teams turn risk assessment from a qualitative documentation exercise into a quantitative design control process - faster, clearer, and more predictable.
Let’s explore if this approach is relevant to your project.
Talk to an expert

By submitting, you accept RD8's Privacy Policy and Terms of Service.
