Engineering out failure in emergency devices (five 9s reliability)
Reliability targets at 99.999% demand more than testing. They require constraint-driven design that builds robustness into the architecture so reliability is proven from the start.

Meeting FDA’s 5-9 reliability expectations is one of the toughest challenges in drug delivery device development. Traditional tools like DFMEA and FTA are valuable - but without a robust underlying design, they often miss the mark.
RD8’s engineering approach to achieving FDA 5-9 reliability
Our methodology focuses on analysing, understanding, and optimising the foundation of the design - embedding robustness into the device itself.
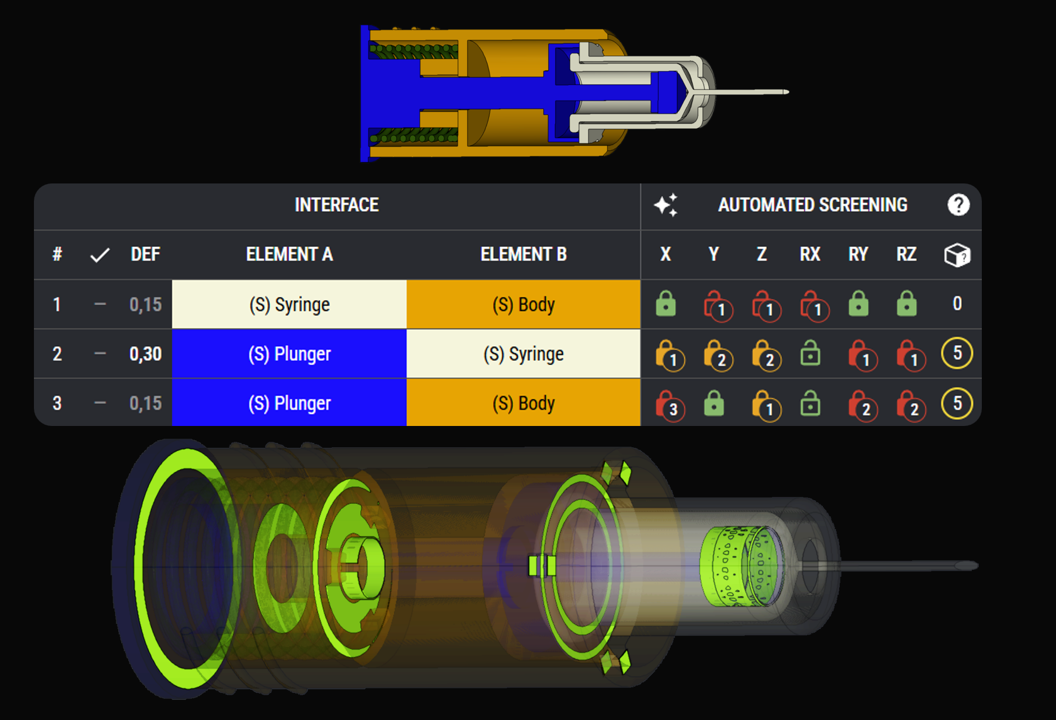
Constraint-Based Design Approach
We focus on interfaces and geometric contact points. We build strong mechanical architectures by identifying all interfaces, functions and load paths. This preserves critical quality attributes (CQAs) like dose accuracy, activation force, and injection time.
By ensuring only intended paths and mechanical interactions occur, they can be optimised to perform under real-world variation. This eliminates redundant or potentially conflicting paths that could compromise reliability.
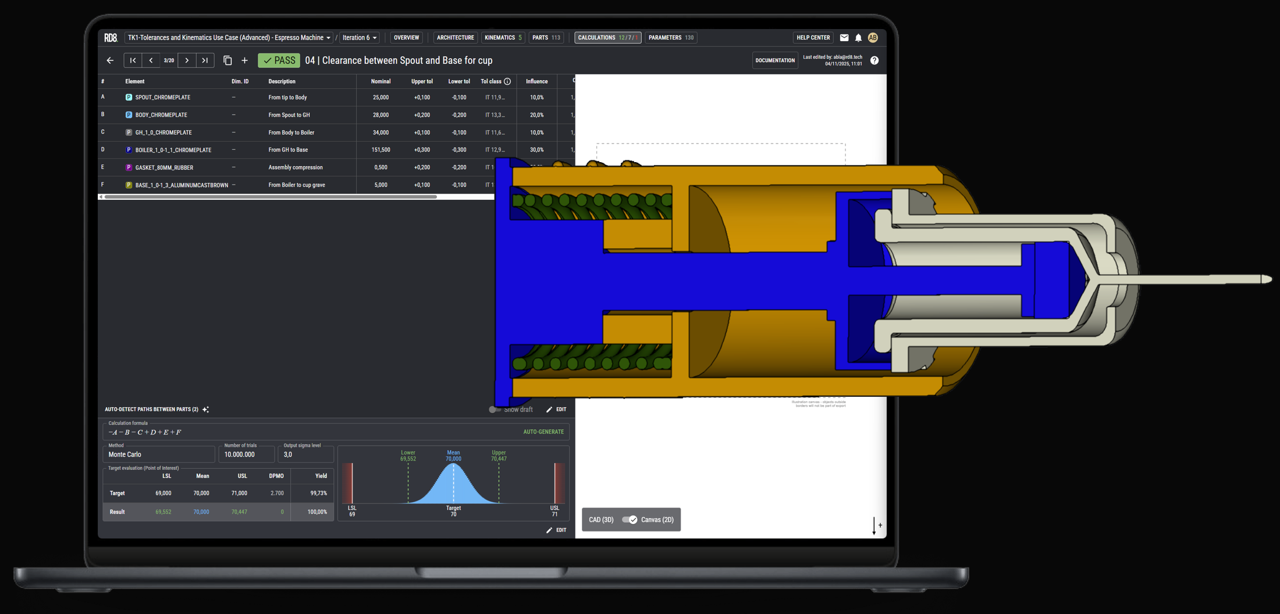
Tolerance-Aware Engineering
We map and model all core functions to expose sensitive transfer functions early. This allows tight specifications only where sensitivity cannot be designed out - easing manufacturability, improving scalability, and keeping CQAs within specification.

Is 5-9 reliability your next challenge?
We bring industry-leading expertise to your challenge - and we tailor a fit to your team. A short conversation will clarify whether RD8’s approach can help.
Let's explore fit
Talk to an expert

By submitting, you accept RD8's Privacy Policy and Terms of Service.
