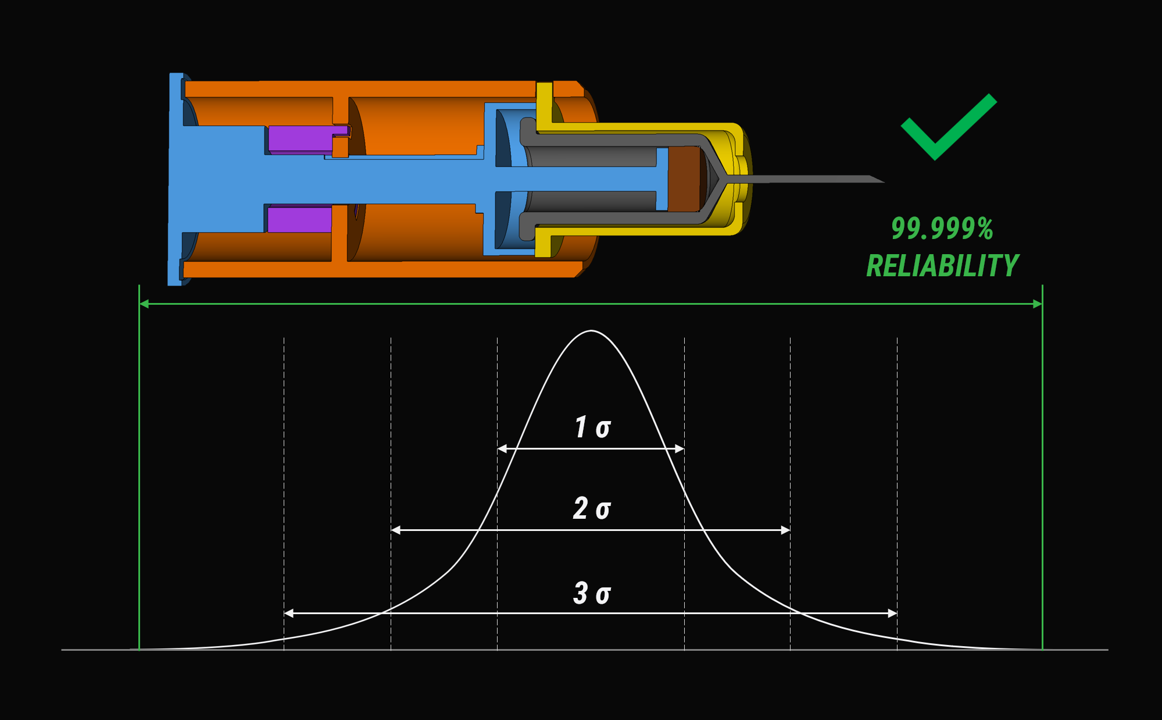
Reliability requirements for emergency-use drug delivery devices, be it ISO or regulatory bodies like FDA are clear: less than one failure in 100,000 uses. The stakes are high.
The industry usually has strong tools for requirements management and system engineering to set up compliant specifications. We also have good and reasonably clear guidelines for testing and proving performance – to some extent straight from relevant regulations and applicable standards.
But here’s the pain point:
- Running large-scale reliability tests is expensive and time-consuming, but a necessity.
- If results fall short, you’re forced into costly iterations, delays, or bolting on extra testing.
This can keep quite a few organisations up at night…
The real gap?
Moving systematically and repeatably from a good, professional specification to a design that passes rigorous testing. Most tools at this stage — FTA, DFMEA, & Reliability Block Diagrams — are often only used retrospectively or in vacuum away from the main design activities. Often these tools are not utilised properly until a mature design is in place at which point they become less powerful because the complexity of altering the final design becomes more cumbersome reducing the overall impact of these activities. That means iteration is almost inevitable, even if you don’t make any real mistakes or oversights along the way.
What’s missing is a framework and tools to bridge the gap—to help design teams engineer reliability into the mechanical design already at conceptualisation. Because the mechanical design is the bridge. The question is: how do we build and dimension it? How do we do it systematically and repeatable?
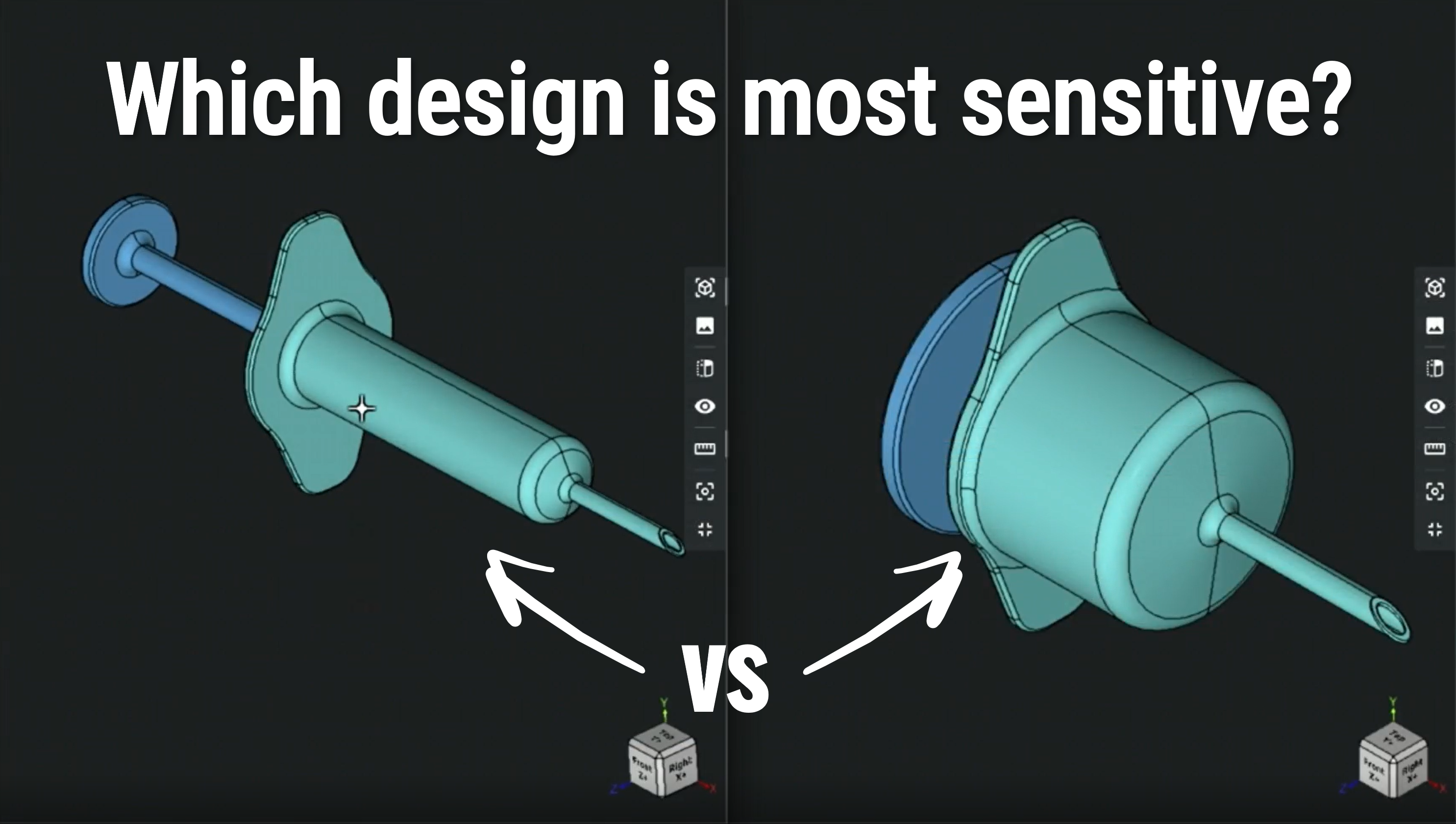
See how the Robust Design mindset (and RD8 tools) aid to predict quality: https://www.rd8.tech/guides/tolerance-stack-up/step-4.
My experience is that industry has a real need here, but a lot of companies are struggling to find their answers.
This topic is something we are hard at work at – we have created a highly operational framework and toolbox from our project portfolio. We would love to get a more structured dialogue between all of us as professionals.
Contact us
Reach out to our Drug Delivery Expert team to get a structured dialogue for how to achieve 5-9 Reliability. The benefits for both companies and patients are real.
By submitting, you accept RD8's Privacy Policy and Terms of Service.